BioHackerLab/Experimental
Hopp til navigering
Hopp til søk

Experiments performed at Bitraf.
05 Jul 2016 - Bitraf PCR #1

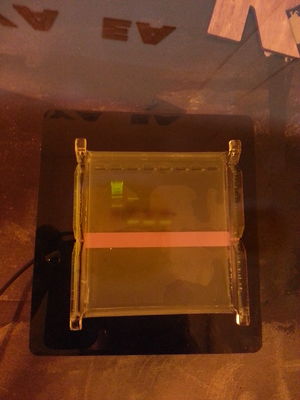
Result from PCR experiment to copy the 5.8S rRNA gene RDN58 and flanking ITS regions from yeast (S. cerevisae). Primers used were ITS1 (TCCGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC). Primers were supplied by Macrogen Inc. Primer target concentration: 0.5 uM each. From left: DSBio 1kb ladder (5 uL), DSBio 50bp ladder (5 uL), PCR sample 1 (10 uL), PCR sample 2 (10 uL), PCR negative control (no template) sample (~5-10 uL). Electrophoresis at 75V for ~45 min on 1 % agarose with GelGreen DNA stain. Visualized with DarkReader DR22 transilluminator. PCR performed 05.07.16 with OpenPCR and DongSheng Biotech Taq mix. Reaction volume 50 uL. Template source is store bought dry yeast (Idun tørrgjær). Template source was prepared by dissolving 0.1 g dry yeast in 10 mL distilled water, and incubating 50 uL of the resulting yeast solution in a PCR tube at 98C for 10 min in openPCR. For PCR sample 1 and 2, 1 uL and 2 uL of the supernatant after incubation was added to the reaction mix, respectively. PCR program was as follows: Initital denaturation: 94C for 3 min. Repeated cycles: Denaturation: 94C for 30s. Anneal: 55.5C for 30s. Extension: 72C for 1 min. Final extension: 72C for 10 min. 35 cycles. Total run time: ~2h 20 min.
10 Jul 2016 - Bitraf PCR #2

Result of shortened PCR program july 10, using 30 cycles and no final extension step for amplification with primers ITS1 and ITS4 and same template as prepared July 5 (stored in freezer). From left to right: DSBio 1kb ladder (5 uL), PCR sample using 1 uL template (10 uL), PCR sample using 2 uL template (10 uL), negative control PCR sample (no template) (10 uL). For the second PCR sample, no PCR product band is visible and the reaction appears unsuccesful.
11 Jul 2016 - Bitraf PCR #3
PCR was performed using the same PCR setup and program as July 10 except for the selection of templates and template amounts. The following templates and template amounts were used:
- #1/HS1A: 1 uL yeast sample #1. (S. Cerevisiae [1])
- #2/HS1B: 2 uL yeast sample #1. (S. Cerevisiae [1])
- #3/HS2A: 1 uL yeast sample #2. (Brettanomyces? [1])
- #4/HS2B: 2 uL yeast sample #2. (Brettanomyces?[1])
- #5/P1: 1 uL S. cerevisiae (Idun tørrgjær. Template prepared 5 july stored in freezer)
- #6/P2: 2 uL S. cerevisiae (Idun tørrgjær. Template prepared 5 july stored in freezer)
- #7/NT: Reaction mix and primers only, no template.
The PCR samples were moved to freezer after completion of the PCR run.
[1]: Liquid cultures provided by Heikki. To prepare template, 50 uL liquid culture was incubated at 98C for 10 min in OpenPCR.